PRODUCTS
- SILICONE SEALANT PRODUCTION LINE
- EMULSION / RESIN PRODUCTION LINE
- ADHESIVE PRODUCTION LINE
- HOT MELT PRODUCTION LINE
- PAINTS & COATINGS PRODUCTION LINE
- POWDER COATING PRODUCTION LINE
- Reactor/Mixing Kettle
- Dispersing Machine
- Emulsifying Machine
- Filters
- Filling& Capping Machine
- Mixing/Storage Tank
- Lab Machine
- Accessories
The field is required.
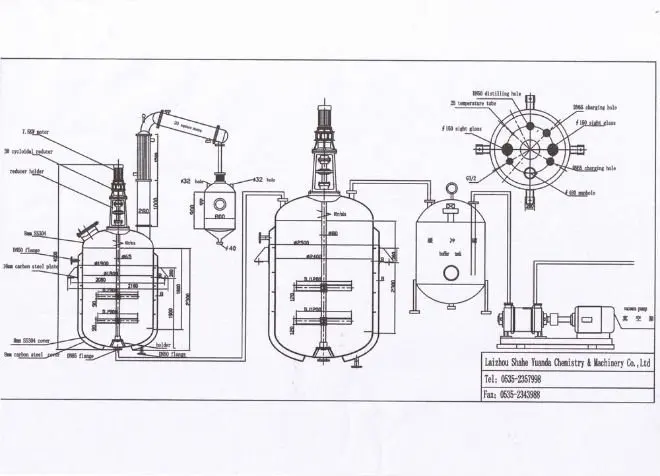
Unsaturated Polyester Resin Full Production Line
Other recommendations
Key attributes
Industry-specific attributes
automatic grade
Automatic
voltage
380V
machinery capacity
5200
Other attributes
key machines
reactor
Weight (KG)
3200
brand name
TGM
place of origin
Guangdong, China
Product name
Epoxy Resin Full Production line
Application
Epoxy Resin alkyd resin
Advantage
High Efficiency Low Cost
Certification
CE ISO
Function
Automatical Production
Capacity
1000-10000L
Type
Production Machinery
Max. Loading Capacity
16000L
Core Components
Motor, Pump, PLC, Bearing, Engine
After-sales Service Provided
1 Year
Packaging and delivery
Selling Units:
Single item
Overview

Semi Automatic unsaturated polyester resin production line in Small and Large Scale Capacity
1. Raw Material Preparation
* Epichlorohydrin (ECH): A common starting material, typically derived from propylene.
* Bisphenol A (BPA): The primary hydroxyl-containing compound used in the reaction.
* Catalyst: Usually, a strong base such as sodium hydroxide (NaOH) is employed as a catalyst to promote the reaction.
* Solvent: Sometimes used to control the reaction conditions.
* Epichlorohydrin (ECH): A common starting material, typically derived from propylene.
* Bisphenol A (BPA): The primary hydroxyl-containing compound used in the reaction.
* Catalyst: Usually, a strong base such as sodium hydroxide (NaOH) is employed as a catalyst to promote the reaction.
* Solvent: Sometimes used to control the reaction conditions.
2.Glycidation Reaction (Epoxidation)
* In this step, epichlorohydrin reacts with bisphenol A under basic conditions (in the presence of sodium hydroxide).
* In this step, epichlorohydrin reacts with bisphenol A under basic conditions (in the presence of sodium hydroxide).
3. Distillation and Purification
* The crude product obtained from the reaction is distilled to remove impurities, unreacted materials, and excess water.
* This step ensures the desired molecular weight and viscosity are achieved for the final product.
* The crude product obtained from the reaction is distilled to remove impurities, unreacted materials, and excess water.
* This step ensures the desired molecular weight and viscosity are achieved for the final product.
4. Control of Molecular Weight
* By adjusting the ratio of bisphenol A to epichlorohydrin, the molecular weight and properties of the unsaturated polyester resin can be controlled.
* Lower molecular weight products result in liquid unsaturated polyester resins (LER), while higher molecular weight products result in solid epoxy resins (SER).
* By adjusting the ratio of bisphenol A to epichlorohydrin, the molecular weight and properties of the unsaturated polyester resin can be controlled.
* Lower molecular weight products result in liquid unsaturated polyester resins (LER), while higher molecular weight products result in solid epoxy resins (SER).
5.End-Capping (Optional)
* Some epoxy resins undergo a process called end-capping, in which the terminal hydroxyl groups are reacted with additional epichlorohydrin to cap the ends, reducing the reactivity of the hydroxyl groups and controlling the resin’s properties.
* Some epoxy resins undergo a process called end-capping, in which the terminal hydroxyl groups are reacted with additional epichlorohydrin to cap the ends, reducing the reactivity of the hydroxyl groups and controlling the resin’s properties.
6. Cooling and Solidification
* After the reaction and purification steps, the resin is cooled.
* Depending on the formulation, it may solidify or remain in liquid form. Solid unsaturated polyester resins are typically cooled further to form flakes or pellets for ease of handling and transportation.
* After the reaction and purification steps, the resin is cooled.
* Depending on the formulation, it may solidify or remain in liquid form. Solid unsaturated polyester resins are typically cooled further to form flakes or pellets for ease of handling and transportation.
7. Packaging and Storage
* Once the resin passes quality control tests, it is packed into drums, containers, or other storage units depending on its form (liquid or solid).
* The resin is stored in a controlled environment to prevent contamination or degradation before it is shipped to customers or used in further formulations.
* Once the resin passes quality control tests, it is packed into drums, containers, or other storage units depending on its form (liquid or solid).
* The resin is stored in a controlled environment to prevent contamination or degradation before it is shipped to customers or used in further formulations.




Our Advantages:
1. Customized Flexible Production Capacity
2. Fully explosion proof machines provided
3. One stop pants production line machines supply
4. With plant panning service
5. Could Provide installation
6. Easy maintenance machines
7. Plenty of paints and coatings production line experience and projects







TGM Machine is professional in chemical machinery and complete production lines,with 19 years of industry experience.We integrate,design,development, production, sales and trading, maintenance together.We are a leader of this industry. Our company has professional R&D design team, strong technical support, skilled production team, reliable after sales service,flexible customized solutions, to meet your various standards and non standard equipment procurement needs.We committed to providing the complete and professional chemical equipmentsolutions.Our Main business scope: Disperser, Bead Mill, Mixer, Reactor, Filling machine, Bag filter, diaphragm pump, Zirconium bead, 3 roll mill, and other accessories.
Our machines help to make paints,coatings inks pigments, dyes, glues, adhesives,spin finish,wood glue,glue for paper conversion,viscous pastes, concrete Admixtures, PCVC solvent cement,resins ,sealants,silicone sealant, putty, lithiumbattery , electronic slurry&paste,pulps,liquid rubber,pesticide,liquid fertilizer, disinfectant,liquid detergent, lotions, shampoo, body wash liquid soap, hand sanitizer,nail polish,gel,creams,and other chemical and cosmetics products.









1.Are you a factory or a trading company? where is your factory?
-We are a real factory. Our factory is in fu'an industrial zone, leliu street, shunde ditrict, foshan city, guangdong province,China.
2. What are advantages of your factory?
-1)Our factory is a leader manufacture of Chemical machine With 19 years of Industry experience. We have a lot Technology Patents & Product Patents;
2) Our factory has Professional R&D Teams, High Technical Supports, Skilled Production Team, Reliable After Sales Service, Flexible Customized Solutions;
3) Our factory is the leader of this industry, named with high and new technology enterprise;
4) Our factory has plenty of industry experience, we could offer you the complete palnt planning.
3.Who are your factory's clients? Could you list some of them?
-AKZONOBEL, JUNTON, PPG, NIPPON, DABAO, BURGER, MAYDOS, RAINBOW, HITECH COATING, CHINA PAINT, CHANGJIANG COATING, TOA, LYNWON PIGMENT, PEONY INKS, TL INKS, ZR INKS, SAKATA INKS, KINGSWOO INKS, DIC INKS, ANLI, ACHILLES, REPOW GROUP, SHINETSU ADHESIVES, JUSHI, XINGLONG PESTICIDES, BYD, CATL...
4.Which countries your machine sold to?
-American Market: America, Canada, Mexico, Brazil; -European Market: UK, Germany, Netherlands, Bulgaria, Italy, Russia; -African Market: South Africa, Algeria, Zambia, Cameroon, Ethiopia, Uganda, Madagascar; -Asian Market: Malaysia, Indonesia, Singapore, Vietnam, Thailand, Kazakhstan, Bangladesh, India, Pakistan, Lebanon, Iraq, Egypt, Qatar, Turkey, Saudi Arabia, UAE;
5.Could your factory provide formula and raw materials service?
-.Basically we are machinery suppliers, not products chemist. For some paint&coatings, silicone sealant, textile printing adhesives or pesticide we could help you find chemist and raw materials from our cooperative clients. Other products we are not sure. But if you need our help, we will try our best to dicuss with our serviced cleints and see if they would like sell to you formula or not.
6.Do you have agent in our country?
-1.No, we ship directly from China to you. our machines generally ship without dismounting. You generally only need to connect electricity, you could use directly. If need installation, we provide detailed installation instruction for full line or we send engineers to help you with paying.
7.What's the payment method?
-We accept T/T and L/C payment.
- Tel:
-
Email:
INQUIRY
Certifications
SUBSCRIBE
INQUIRY